Clinical Evidence for L-MethylFolate
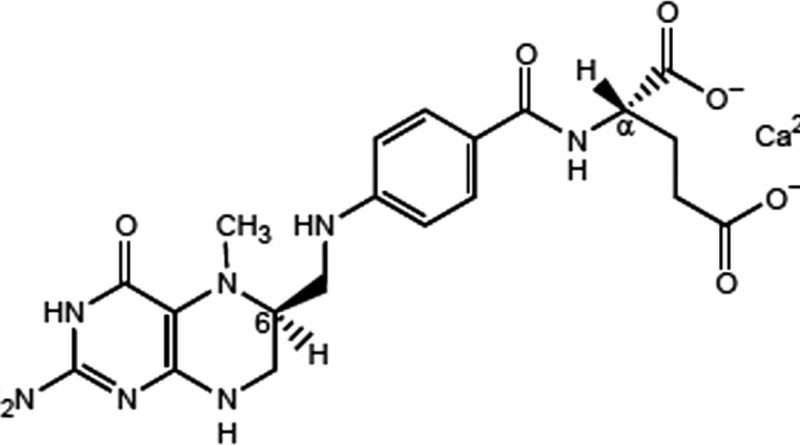
The measurement definition of L-methylfolate (Deplin®, AlphaSigma USA, Inc .; Covington, LA) is a clinically recommended dietary dose of 15 mg/day for use under physician supervision for grief management, and it is particularly healthy. Prerequisites for Depression Patients. The efficacy of this scheme of L-methyl folate, used as an adjunct for the treatment of MDD, has been double-tested in clinical preliminary control of blind, randomized, simulated treatment at any given time.
Best-planned clinical preliminary results for MDD provide strong evidence of the safety and efficacy of the various components of L-methylfolate and its potential role in the treatment of MDD. Furthermore, the remarkable discoveries made from these tests provide significant insight into the fact that those who come up can achieve ideal results and benefit greatly from L-methylfolate treatment.
In particular, the supplemental use of L-methylfolate is effective and is not set in stone to serve in a specific subset of patients, including those with SSRI-safe depression. In general, adverse events with L-methylfolate and Sham treatment can be compared with minimal changes in weight, back, and standing pulse, and prostatic and standing diastolic and systolic circulatory tension.
In a post-hack test, the changes from gauge to 28-Thing HDRS-28 scores were even more significant in plasma markers with fully identified plasma markers, including L-methyl folate versus sham treatment, including SAMe: SAH (S-adenosylhomocysteine). Added. The ratio of less than 2.71, major high-impact CRP (hs-CRP) levels of 2.25 mg / l and higher, and blood levels of 3.28 g / mL or lower (p ≤ 0.05) 4-hydroxy-2. Most hereditary markers (p< 0.05) in the L-methyl folate bunch have extracellular mass elevations from the norm.
Subgroups of patients with the MTR 2756 Ag / GG or MTR 66 Ag / GG genotypes had significantly more significant changes in HDRS-28 scores than the benchmark in those with the homozygous dominant genotype (p 0.05). A mixture of biomarkers and additional genealogical markers (e.g., MTHFR 677 CT / TT + MTR 2745 AG / GG) has different effect estimates, although the HDRS-28 scores are related to stamped approval (p ≤ 0.05; numbers are expected to behave together) NNT], 1–4). Another post-Hawk investigation by Shelton did not confirm whether patients with an elevated degree of BMI of 30 kg / m2 or IL-8
(p = 0.025) had a more prominent clinical association with auxiliary L-methylfolate. Responses have been received. Shortly after the measurements, the addition of an increased degree of IL-8, IL-12, TNF-α, hsCRP, or leptin (p < 0.03) demonstrated a pooled effect on patients with a BMI of 30 kg / m2.
Clinical scenario for L-methylfolate use in MDD
Preliminary positive results for the use of L-methylfolate as an adjunct therapy for grief suggest that specific patient symptoms may be particularly prevalent in response. In addition to the superior and optimal conditions under which L-methylfolate use is more responsible for the patient’s side effects, these symptoms are up to date.
Good position
Broadly speaking, L-methyl folate and a variety of folates have been identified as auxiliary mediators for optimal remission in patients with MDD and are of general medical benefit. Those who prefer nutritious items with prevented side effects may find L-methylfolate an attractive helpful option, which can be done by people who try an extensive routine including an exercise list to monitor for side effects with a more sensitive load. Patients with different responses to SSRIs / SNRIs may be treated with L-methylfolate to achieve better results. In one review study, patients with elevated L-methylfolate with SSRI / SNRI had higher elevations in burden expression (without adjustment for SSRI / SNRI secondary effects) than patients treated with SSRI / SNRI alone. Treated with P = 0.01). Profile.
Better position
MD-treatment L-methylfolate may have a better clinical benefit in patients with evidence of depression, especially with SSRIs. As shown above, the use of L-methylfolate in patients with SSRI-safe inactivation is associated with improved enrichment. These results are particularly effective in patients with MTR 2756 AG / GG genotype, low EQ: SAH ratio, high BMI, or increased CRP or IL-8. Depending on treatment considerations SSRI may include a variety of antipsychotics in depression, which may increase the risk of tardive dyskinesia, perhaps irreversibly important in these studies. Differential antipsychotics may also be abnormal in large patients due to secondary effects of group-related metabolism (i.e. weight gain; insulin, glucose, and low-fat lipoprotein cholesterol; diabetes mellitus; and hypertriglyceridemia). The use of these may put patients at greater risk for cardiac contusions, including coronary disease, which are additionally associated with higher CRP levels.
Best position
Decreased folate or its metabolism has been reported in patients with MDD and BMI 30 kg / m2, as well as impaired MTHFR protein activity, which is a reasonable use of adjuvant use of L-methylfolate. In some patients, low folate levels can be caused by alcohol abuse, hypothyroidism, dietary problems, pregnancy, gastrointestinal problems, or taking certain medications.
Therefore, physicians should add L-methylfolate to the treatment routine of patients with any of these factors. Clinical preliminary post hack testing with L-methyl folate showed the largest effect size for NNT = 1 vs. Sham treatment. In patients whose SSRI neglected to respond to treatment focused on 2 biological markers of L-methylfolate, irritation/crapulence and additionally on folate digestion quality polymorphisms (e.g., MTR2756AG / CG + COMT) rs 4633) cc or BMI 30 kg. Furthermore, coagulation effects have been demonstrated in patients with a BMI of 30 kg / m2 due to the added degree of IL-8, IL-12, TNF-α, hsCRP, or leptin. Therefore, more than 1 specific piece of evidence in these variables is a particularly important indication that auxiliary L-methylfolate use may be acting on a patient’s clinical outcome.